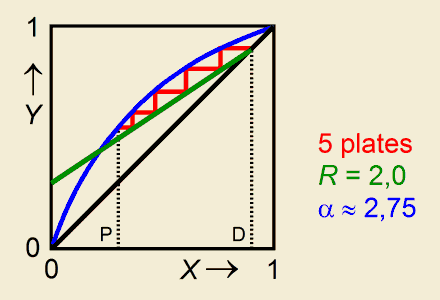
Distillation in a column with a given number of theoretical platesThe boiling liquid is a mixture of two volatile components, wherein the mole fraction of the more volatile component is symbolized by X (hence 1–X matches the less volatile component).
|
Program Listing |
Elucidation and Help |
| PROGRAM:RECTIBD | Title |
| ClrHome | > PRGM > I/O > ClrHome |
| Disp "REL.VOLATILITY" | (α>1) |
| Input A | |
| max(A,1)→A | > MATH > NUM > max( "→" Symbolizes the STO> key |
| Disp "X POT MIXTURE" | Composition of the boiling mixture. X is the mole fraction of the more volatile component of the liquid (0<X<1). |
| Input M | |
| min(M,1)→M | |
| Disp "NUMBER OF PLATES" | Number of theoretical plates inclusive of the pot |
| Input P | |
| Disp "REFLUX RATIO" | |
| Input R | |
| {P,3}→dim([A]) | > 2nd, MATRX > MATH > dim( > 2nd, MATRX > NAMES > [A] |
| 1→B:M→C | |
| ClrHome | |
| Lbl 1 | > PRGM > CTL > Lbl Beginning of iteration loop |
| If B–C<.00001 | |
| Goto 2 | |
| (B+C)/2→D:D→X | |
| For(N,1,P) | |
| (RX+D)/(R+1)→Y | Y is the mole fraction of the more volatile component of the vapour (0<Y<1). |
| Y/(Y+A(1–Y))→X | Y and X are at equilibrium |
| N→[A](N,1) | |
| X→[A](N,2) | |
| Y→[A](N,3) | |
| End | |
| If X<M | |
| Then | |
| D→C | |
| Else | |
| D→B | |
| End | |
| Goto 1 | |
| Lbl 2 | |
| round((B+C)/2,2)→D | |
| Disp "X DISTILLATE",D,"","PLATE, X, Y >>" | Distillate composition |
| Pause | |
| round([A],2)→[A] | |
| Disp [A] | Compositions (first seven plates) |
| Pause :ClrHome | |
| Disp "X,Y IN MAT [A]" | Merely a reminder |
After running and quitting the program, you still can have access to the liquid and vapour compositions X and Y of all plates. This may especially be of intrest when the number of plates exceeds seven. You'll find them stored in matrix [A] (> 2nd, MATRX > NAMES > [A], ENTER, ENTER).
ExampleConsider a mixture of benzene and toluene. Input values: Output: The vapour with composition Y3 = 0,88 is ascending from plate 3. (To retrieve the plate compositions as shown in an example coming with the program RECTIBP, put in XP = 0,58.) |
Hein ten Horn
Top | Programs Texas Instruments | Home